Intervention and Trial
ProjectInterventionTrialThe Bump2Baby and Me Project
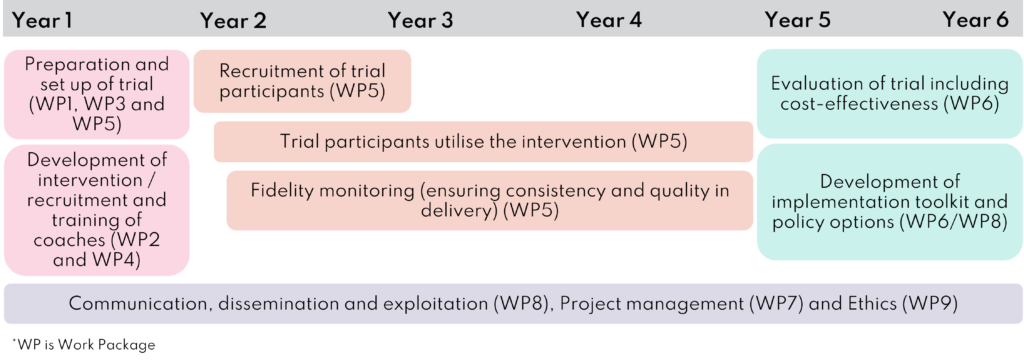
Impact Diabetes Bump2Baby and Me (IDB2B) was a five-and-a-half-year research project that ran from January 2019 to June 2025. The project consisted of three key elements:
- the development of the Bump2Baby and Me mHealth Coaching Programme and setting up of the randomised controlled trial (Year 1)
- implementation of the randomised controlled trial (Year 2-4)
- evaluation of the randomised controlled trial including its cost effectiveness, and the development of a toolkit and policy options (Year 5-6).
Led by University College Dublin, the consortium of nine partners brought together expertise in implementation science, lifestyle change, health psychology, mHealth technology, health economics and health service delivery. An External Advisory Board also provided independent scientific, policy and ethics advice and support to the consortium.
Four main outcomes were expected from the project:
- the Bump2Baby and Me mHealth Coaching Programme;
- an implementation toolkit
- economic model, and
- policy options.
Our Project Partners

University College Dublin
UCD is the Project Coordinator and the leader of WP6, WP7 and WP9.

University of Copenhagen
The University of Copenhagen is leading WP4 and WP5, developing training resources and monitoring the running of the research trial.

Deakin University
Deakin University are contributing to WP2, co-creation of the app, and the project evaluation in WP8.

University of Bristol
The University of Bristol is the UK trial site and will lead the implementation planning work for the EU study.

Monash University
Monash University is leading WP1 and the clinical lead for the Australian trial site.

Liva Healthcare
Liva is WP2 leader and is responsible for the creation and development of the Bump2Baby and Me app.

Beta Technology
Beta is leading WP8, coordinating all communication, dissemination and exploitation activity for the project.

University of Granada
The University of Granada will contribute to research activities across all work packages and translate the app content into Spanish.

Aarhus University
Aarhus University’s main role is to supporting activities related to health literacy, and support the dissemination of health literacy results.

Bump2Baby and Me mHealth Coaching Programme
The innovative Bump2Baby and Me mHealth Coaching Programme intervention targets women at risk of developing gestational diabetes. The programme provides personalised, evidence-based, healthy eating and exercise support during pregnancy and the first year after birth through a smartphone app and real-life health coach. This intervention is novel because it:
- is a tailored mHealth solution provided by a health coach, not artificial intelligence
- can be provided remotely
- sits alongside the local healthcare system
- can be adapted to suit local service requirements (e.g. language), and
- has the potential to bring efficiency and cost effectiveness.
The right support at the right time
Women can be supported to better self-manage their modifiable risk factors through the health coaching and the app. Trained health coaches can optimise engagement with the app and help to personalise content for each woman and her baby, ensuring they only received the right information at the right time. This behaviour change intervention was developed to support through pregnancy (bump) to birth (baby) and for 12 months postpartum. All of the health coaches were trained and provided with a health coach manual.
The Bump2Baby and Me Randomised Controlled Trial
The Bump2Baby and Me randomised controlled trial (Trial registration: ACTRN12620001240932) ran in maternity hospitals in Ireland, the UK, Spain and Australia (Dublin, Bristol, Granada and Melbourne). The trial focused on working with women who were identified at risk of gestational diabetes and who would therefore benefit most from this behaviour change intervention.
A total of 865 pregnant women who were identified at risk of developing gestational diabetes via the Monash GDM Screening Tool1 participated in the trial. The women were randomly assigned to either usual care (control group) or usual care plus the Bump2Baby and Me mHealth Coaching Programme (intervention group).
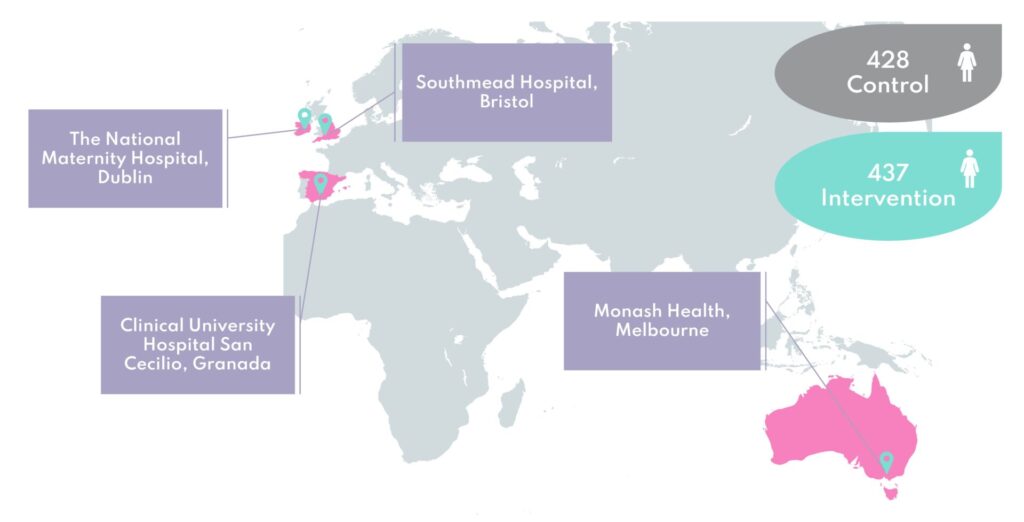
The trial began in early 2021 and recruitment of women to the trial finished in April 2022, exceeding a target of 800 participants. The trial ran until all babies born were one year old, and completed in September 2023.
The Bump2Baby and Me trial collected a wide variety of information including:
- the health of mothers and babies involved
- how the project fitted within maternity services
- how much it cost, and
- what the women thought about the intervention.

Outcome measurements
The trial’s primary outcome was a reduction of 0.8 kg/m2 maternal BMI in the intervention group (at 12 months postpartum). The secondary outcomes included the differences in the following measured during pregnancy and postpartum:
- gestational weight gain and weight status
- maternal blood pressure
- maternal physical activity and sleep
- maternal psychological health
- maternal and infant diet
- metabolic markers including blood glucose and blood lipids
- glycaemic status and GDM diagnosis
- birth data including mode of delivery, birth weight, placental weight, and any complications
- newborn and infant anthropometry (weight centiles, BMI z-scores)
- breastfeeding (any and exclusivity) and duration
- infant development
- infant physical activity and sedentary time.
The results of the trial are outlined in the Bump2Baby and Me toolkit.
References
1Teede HJ, et al. Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol. 2011 Dec;51(6):499-504.