Project Progress Update
Any collaborative research project owes some of its success to being able to implement and organise the project team around a clear, feasible and ambitious project plan. The heart of the Impact Diabetes B2B project plan is the ‘Bump2Baby and Me’ randomised controlled trial (RCT), which tests an innovative, personalised health coaching application and screening process, and its suitability for delivery within routine health service care. The project’s ultimate objective is to help prevent maternal and child diabetes, overweight and obesity and other non-communicable associated diseases.
It is important to regularly review project plans to ensure they are still on track and delivering what they set out to do. In the case of Impact Diabetes B2B, the European Commission (EC), our main funder, asks for periodic reports every 18 months. We recently completed our second reporting period, and the report was submitted on time, thanks to the efforts of all partners, at the end of February 2023. The EC also conduct a formal project review as part of the periodic reporting evaluation. Our review took place in the middle of March, and we were delighted to receive positive feedback.
Our project achievements
- The collection, extraction, and synthesis of all current evidence from the literature to inform the development of the Bump2Baby and Me RCT. This resulted in an integrated antenatal and postnatal framework to support the trial implementation.
- A co-created Bump2Baby and Me RCT version of the LIVA application and platform was developed with a comprehensive health coaching manual and content library in both English and Spanish.
- A mapping protocol and tool for the Exploration, Preparation, Implementation and Sustainment (EPIS) framework assessment was developed and applied to the contextual mapping of sites, which analysed the factors with the potential to influence the intervention implementation.
- Qualitative interviews with key healthcare professionals and an online survey of antenatal and postnatal maternity services healthcare professionals in each country were conducted. They sought to gather information on standard practice and protocols for identifying and managing women with GDM and ensuring appropriate GWG. Survey data analysis and dissemination plans are underway.
- Ethical compliance and fidelity monitoring processes were established and will continue for the trial duration to ensure the RCT is implemented as intended.
- The target number of women recruited to the RCT at the four clinical sites in Ireland, the UK, Spain and Australia was achieved. There is one group receiving the intervention and the other receive usual care. 727 babies were born and over half of 12-month final visits are completed so far. These numbers are important to understand whether the study was delivered as planned and is on schedule.
Percentage of Participants in each Study Stage (postpartum)
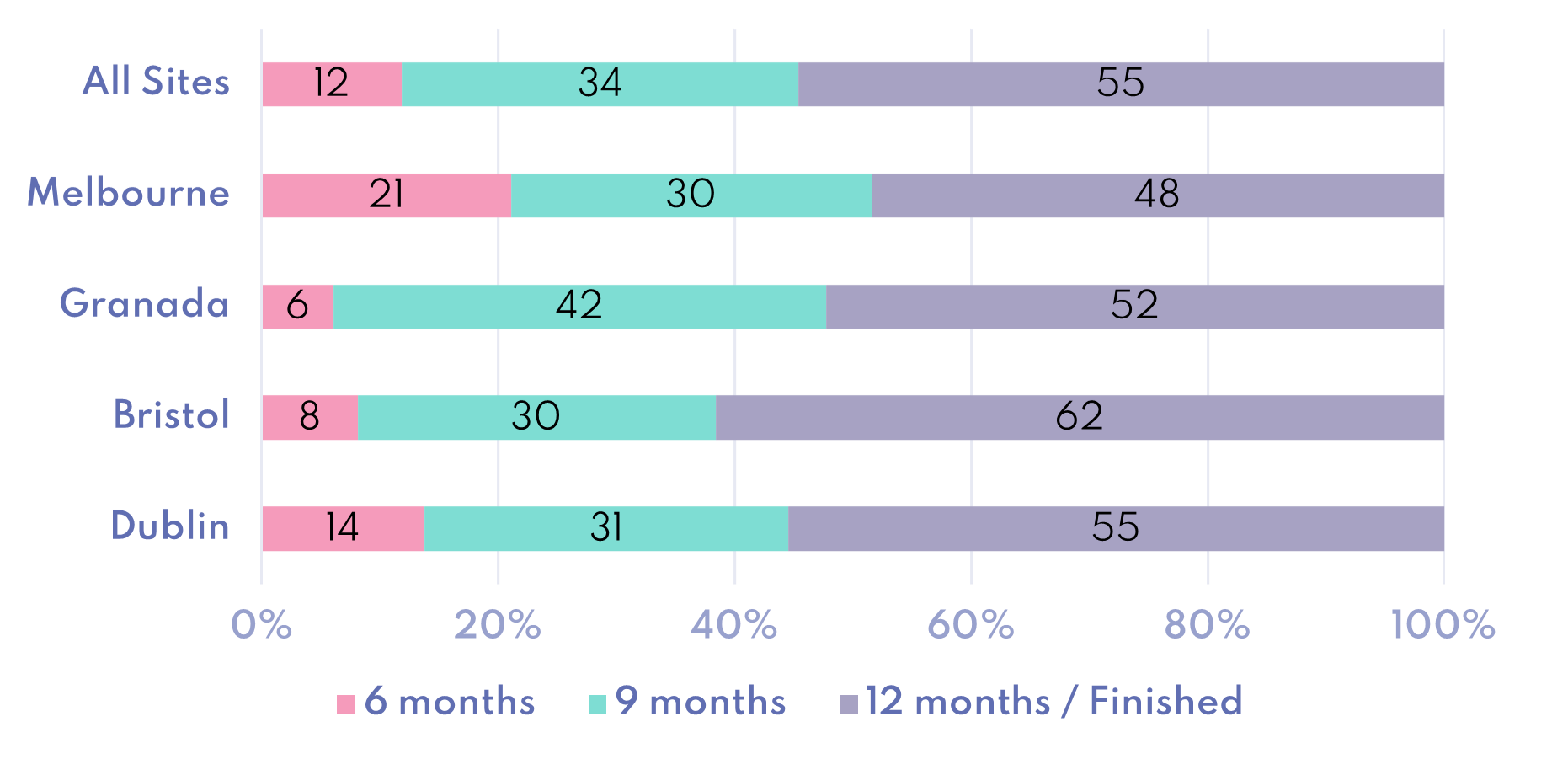
- The baseline, pregnancy and birth data collection are complete. This is being cleaned so that analysis can start on it. Postpartum data collection is ongoing.
- Partners presented at several conferences to raise awareness of the project and the RCT. Five papers were published, including the Bump2Baby and Me RCT Protocol in Trials journal.
- Three successful implementation forums were held to facilitate engagement with policy and healthcare stakeholders.
In conclusion, we have made excellent progress despite all the challenges faced because of the COVID-19 pandemic. This is credit to the clinical research teams involved and their extraordinary efforts to keep the RCT on track. The RCT will run until September 2023, when all participants will have completed their 12-month visits. In the remaining part of the project, we will focus on evaluating the evidence from the randomised controlled trial including its cost effectiveness and developing an implementation toolkit and policy options.