High quality, evidence-based research to guide decision making
The Bump2Baby and Me randomised controlled trial (RCT) will test a novel healthcare intervention during pregnancy and the first year after birth, which is the Bump2Baby and Me mHealth coaching programme. With all trials, it is important to know if it works but equally it is important to look at how it works. To do this we need to examine how the trial is implemented and for Bump2Baby and Me we are using the RE-AIM (reach, effectiveness, adoption, implementation, maintenance) framework. This framework was developed to improve the translation of research into practice. We will use this framework to measure:
- Reach – how well we recruited the women we wanted to recruit;
- Effectiveness – how well the trial worked;
- Adoption – how well staff engaged with screening;
- Implementation fidelity – how well the programme was delivered; and
- Maintenance – how well practices were kept up over time.
The evidence we gather, both from the trial and on its implementation, will also contribute to the TOPCHILD Collaboration. TOPCHILD (Transforming Obesity Prevention for Children) is an exciting Collaboration, funded by the National Health and Medical Research Council of Australia. It brings together researchers from around the world to transform the thinking and practices around early childhood obesity prevention.
The Bump2Baby and Me RCT and TOPCHILD Collaboration will provide high quality, and robust evidence to inform healthcare and policy decision making. The Bump2Baby and Me project has been designed to test and develop the mHealth coaching programme so that it will be suitable for rollout across healthcare systems, supported by an implementation toolkit and economic justification for adoption.
But where does this research sit in terms of the quality of evidence it provides?
Each research study design has advantages and disadvantages, and researchers spend a lot of time selecting the right one. However, it is widely accepted that there is a hierarchy in terms of the quality of evidence they provide. The figure below outlines some of the different types of study and where they sit.
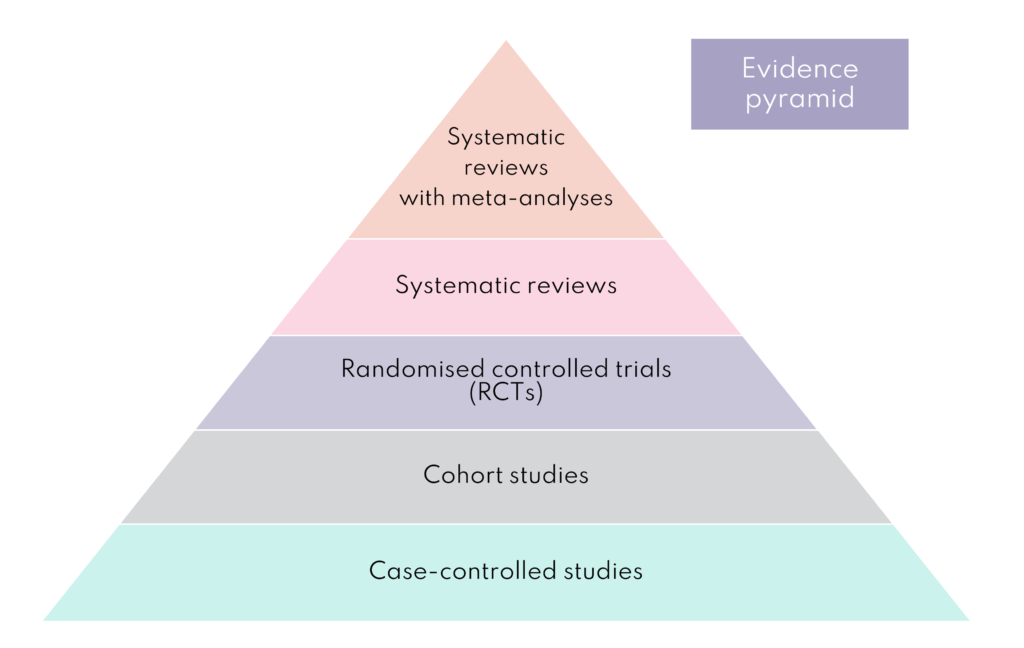
Randomised controlled trials
Randomised controlled trials (RCT) are prospective studies following participants forward in time. This is an important feature as the outcome is not known, unlike in retrospective studies which look back as this introduces bias. RCTs measure the effectiveness of a new intervention or treatment and participants are recruited and randomly assigned to two or more groups. In an RCT with two groups, the experimental group receives the intervention being tested and the comparison or control group receives an alternative intervention, a dummy intervention (placebo) or no intervention at all. Bump2Baby and Me has two groups of participants; the intervention group who receive the mHealth coaching programme alongside usual antenatal care and the control group who receive usual antenatal care.
Systematic reviews and meta-analysis
Systematic reviews can provide a summary of all available evidence to answer specific pre-defined research questions, e.g., how effective are health coaching programmes in pregnancy for women with diabetes? In simple terms, a systematic review is research synthesis, and they are considered the most reliable sources of evidence to guide clinical practice and decision-making.
Systematic reviews will commonly include a meta-analysis, which is a statistical method to combine and analyse the outcome data from each of the studies included in the systematic review. By combining the data in the meta-analysis, researchers can see if the overall pattern of outcomes are in favour of a real effect or not. The meta-analyses are situated at the top of the ‘evidence quality pyramid’ because they produce more reliable findings.
The TOPCHILD Collaboration takes this quality even further as it is collecting individual participant data (IPD). This means that de-identified row-by-row data will be combined across trials, rather than a summary group result which is what is typically used for meta-analysis. This enables more detailed analyses, in particular the ability to evaluate the impact of individual participant level characteristics. The TOPCHILD Collaboration will also be deconstructing interventions into their components to determine which elements of interventions are most effective. This will provide a high-quality systematic review with more efficient and collaborative research that reduces research waste and bias. TOPCHILD will be the largest individual IPD meta-analysis evaluating behavioural interventions for the prevention of early childhood obesity to date.
Bump2Baby and Me will provide its outcome data to the TOPCHILD Collaboration.
Publications
Bump2Baby and Me protocol:
O’Reilly SL, Burden C, Campoy C et al on behalf of the IMPACT DIABETES B2B Collaboration Group. Bump2Baby and Me: protocol for a randomised trial of mHealth coaching for healthy gestational weight gain and improved postnatal outcomes in high-risk women and their children. Trials 22, 963 (2021). doi:10.1186/s13063-021-05892-4
TOPCHILD has published two protocols:
Hunter KE, Johnson BJ, Askie L, Seidler AL et al on behalf of the Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration. Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration: protocol for a systematic review with individual participant data meta-analysis of behavioural interventions for the prevention of early childhood obesity. BMJ Open 2022;12:e048166. doi: 10.1136/bmjopen-2020-048166
Johnson BJ, Hunter KE, Golley RK, Seidler AL et al on behalf of the Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration. Unpacking the behavioural components and delivery features of early childhood obesity prevention interventions in the TOPCHILD Collaboration: a systematic review and intervention coding protocol. BMJ Open 2022;12:e048165. doi: 10.1136/bmjopen-2020-048165